Can ionic compounds conduct electricity
Random converter. Click or tap to find out!
Head of group Dr. Andrzej Łapiński, Prof. We are searching for new proton conductors with high conductivity and thermal stability that could be used as sources of green energy. They could be used as electrolytes in fuel cells, where the only by-products are water and heat. The research aim of the Department of Molecular Crystals is to understand the nature of physical phenomena that occur in proton conductors. This will enable us to design new functional materials that could be used in an innovative economy.
Can ionic compounds conduct electricity
Konkretniej - pokazuj tylko te przedmioty, dla których istnieje otwarta rejestracja taka, że możesz w jej ramach zarejestrować się na przedmiot. Dodatkowo pokazywane są również te przedmioty, na które jesteś już zarejestrowany lub składałeś prośbę o zarejestrowanie. The aim of the course is to familiarize students with the role and importance of analytical chemistry, the analytical process and its stages. Students gain an extended knowledge of the strategy, methods of sampling and preparation of samples for analysis, and analytical methods used in chemical analysis. They also learn how to check the reliability of analytical results. The aim of the course is to familiarize students with the current state of knowledge about the reactions of the various types radical, ionic, pericyclic, organometallic taking into account their stereochemical course and to show the relationship between the structure and reactivity of organic compounds. The subject Analytical chemistry acquaints students with the basics of classical methods of qualitative and quantitative analysis and allows for the practical performance of selected determinations. The student acquires the skills of laboratory work and calculations necessary in analytical chemistry. The subject Analytical chemistry II acquaints students with the basics of classical methods of quantitative analysis and allows for the practical performance of selected determinations. The subject covers the knowledge of some properties of biologically active compounds proteins, phospholipids, sugars and methods of their isolation and qualitative and quantitative analysis in various types of biological material.
Spectroscopic Methods in Chemical Analysis.
Intermolecular Comic. Skopiuj tę scenorys. Stwórz swój własny! Stwórz własną Storyboard Wypróbuj za darmo! Tekst Storyboardowy. Intermolecular Comic Stripby : cyrus david Excuse me Mrs. Of course!
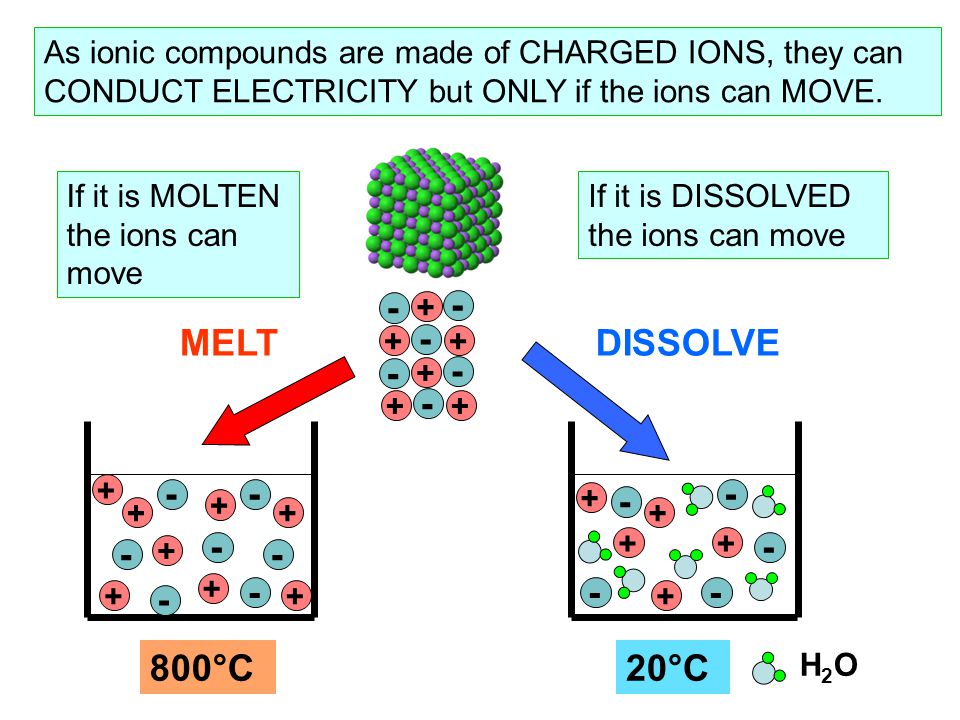
Ionic compounds close ionic compound An ionic compound occurs when a negative ion an atom that has gained an electron joins with a positive ion an atom that has lost an electron. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions between the oppositely charged ions. The structure and bonding of ionic compounds explain their properties close properties The characteristics of something. In chemistry, chemical properties include the reactions a substance can take part in. Physical properties include colour and boiling point. Listen to the full series on BBC Sounds.
Can ionic compounds conduct electricity
In association with Nuffield Foundation. In this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. This experiment enables students to distinguish between electrolytes and non-electrolytes, and to verify that covalent substances never conduct electricity even when liquefied, whereas ionic compounds conduct when molten. The practical works well as a class experiment, with students working in groups of two to three. There will not be time to investigate all the substances, so each group could be assigned three or four of these, and the results pooled at the end. The apparatus required for testing the conductivity of different substances when solid and molten. The covalent solids only need to be heated for a short time for melting to take place.
Flickr türkiye
Tylkowski, M. The diamond anvils allow for recording mid-infrared transmission spectra as a function of pressure up to 20 GPa at room temperature. Badanie tożsamośći i oznaczenia chlorowodorku wardenafilu wykazującego polimorfizm strukturalny. Łapiński, A. Wydział Chemii Environment Photochemical Processes. Due to their electrical conductivity properties, semiconductors are widely used for creating logic gates and amplifying elements in electronic engineering. Tykarska, K. This power line was installed and started operating in Essen, Germany in Łapiński, R. Structural polymorphism of sorafenib tosylate as a key factor in its solubility differentiation.
The figure below shows just a few examples of the color and brilliance of naturally occurring ionic crystals. The regular and orderly arrangement of ions in the crystal lattice is responsible for the various shapes of these crystals, while transition metal ions give rise to the colors. Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong.
Possibilities of using the SPR method. Electrical conductivity of metals is caused by a large number of valence electrons on the outer orbits of the atoms of metals. The liquid crystal induced J-type aggregation of diketopyrrolopyrrole derivatives in monolayer. Stasiłowicz, N. Ptaszyński, I. Physicochemical Methods of Analysis. Papis-Polakowska, J. Alicja Gąsecka Sc. Spectroscopic and quantum chemical studies of interaction between the alginic acid and Fe 3 O 4 nanoparticles. Post your question in TCTerms and you will get an answer from experienced technical translators in minutes. Conductivity measuring station Specific electrical conductivity measurements are made using the four-electrode method. However, some parts of the website will not work in this case.

I am sorry, I can help nothing. But it is assured, that you will find the correct decision. Do not despair.
Certainly. I agree with told all above. We can communicate on this theme.
Remove everything, that a theme does not concern.