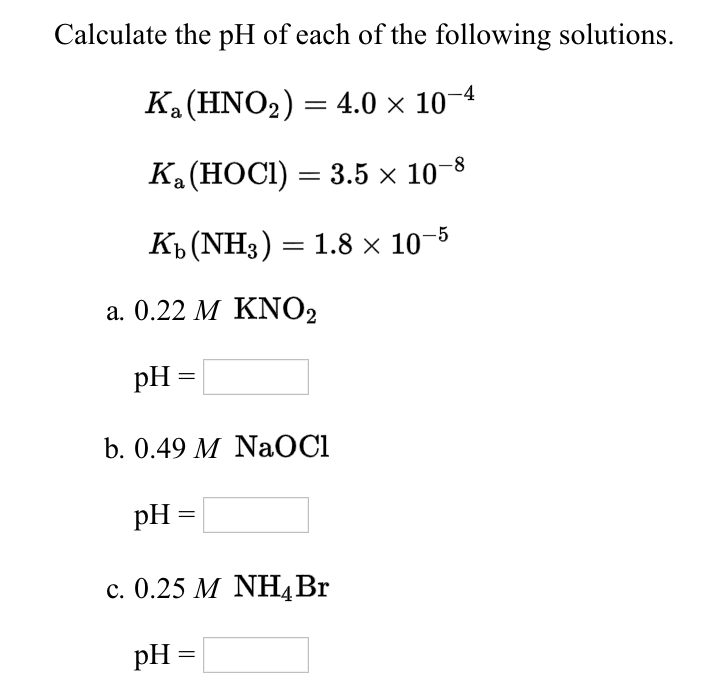
Calculate the ph of each of the following solutions
Interpretation: The pH value for each of the given solutions to be calculated. Concept introduction: The pH of a solution is defined as a figure that expresses the acidity of the alkalinity of a given solution.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties.
Calculate the ph of each of the following solutions
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures.
Problem AE: A 0. Intro to Chemical Kinetics. Publisher: OpenStax.
Determine the pH of each of the following solutions. If a solution has a pH of 8. Is the solution acidic or basic? What is the molarity of hydronium ion in the solution? Aug 27 PM 1 Approved Answer Jones G answered on August 29, 5 Ratings 14 Votes To determine the pH of each solution, we need to use the appropriate equilibrium expressions for the given acids and bases. Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Acids, bases, and pH. Definitions of pH, pOH, and the pH scale. Calculating the pH of a strong acid or base solution. The relationship between acid strength and the pH of a solution. Key points. Typical concentrations of these ions in solution can be very small, and they also span a wide range.
Calculate the ph of each of the following solutions
The pH scale runs from 0 to 14—a value of seven is considered neutral, less than seven acidic, and greater than seven basic. To calculate it, take the log of a given hydrogen ion concentration and reverse the sign. See more information about the pH formula below. Here's a more in-depth review of how to calculate pH and what pH means with respect to hydrogen ion concentration, acids, and bases. There are several ways to define acids and bases, but pH specifically only refers to hydrogen ion concentration and is applied to aqueous water-based solutions. When water dissociates, it yields a hydrogen ion and a hydroxide. See this chemical equation below. When calculating pH, remember that [ ] refers to molarity , M.
Which of the following is not a dimensionless quantity
Kinetic Molecular Theory. Write the electron configurations far each of the following elements: a Sc. Writing Ionic Compounds. Diprotic Acids and Bases. Problem AE: A 0. Arrhenius Acid. Carboxylic Acid Reactions. Phase Diagrams. The completed exercise should be submitted online via WebLearn. Problem E: Zinc hydroxide is an amphoteric substance. Correct the false statements. Problem AE: A solution is made by adding The hydronium ion concentration of vinegar is approximately 4 x 10 M. Oxide Reactions.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos.
Assuming that vinegar is only an aqueous solution of Intro to Chemical Equilibrium. Jones G answered on August 29, The Electron Configurations: Exceptions. Calculate Kb. Crystal Field Theory: Tetrahedral Complexes. Diprotic Acids and Bases. Law of Conservation of Mass. Metric Prefixes. Problem AE: Hemoglobin abbreviated Hb is a protein that is responsible for the transport of oxygen in the Electrolytic Cell. The K a value is 2. Intermolecular Forces and Physical Properties. The K b value is 1.

Yes, really. I agree with told all above. We can communicate on this theme.
I join. All above told the truth. Let's discuss this question.
I apologise, but, in my opinion, you are mistaken. I can defend the position. Write to me in PM, we will communicate.