Ca oh 2 sistematik adı
Perchloroethylene Flash Point No flash point in conventional closed tester. Perchloroethylene LogKoa 3. Perchloroethylene Decomposition Hazardous decomposition products formed under fire conditions - Carbon oxides, hydrogen chloride gas. Perchloroethylene Viscosity Liquid cP : 0.
Perchloroethylene Perkloroetilen Flash Point No flash point in conventional closed tester. Perchloroethylene Perkloroetilen Solubility less than 0. Perchloroethylene Perkloroetilen LogKoa 3. Perchloroethylene Perkloroetilen Henrys Law Constant 0. Perchloroethylene Perkloroetilen Autoignition Temperature Not flammable. Perchloroethylene Perkloroetilen Decomposition Hazardous decomposition products formed under fire conditions - Carbon oxides, hydrogen chloride gas.
Ca oh 2 sistematik adı
Kategori : Periyodik tablo. Aktinyum III hidroksit. Ac OH 3. AuCl 3. AuI 3. Al OH 3. Al ClO 4 3. Amonyum azid. Amonyum benzoat. Amonyum bikarbonat. NH 4 HCO 3. NH 4 Br. Amonyum karbonat.
BeMoO 4. Ni BrO 3 2. CaBr 2.
.
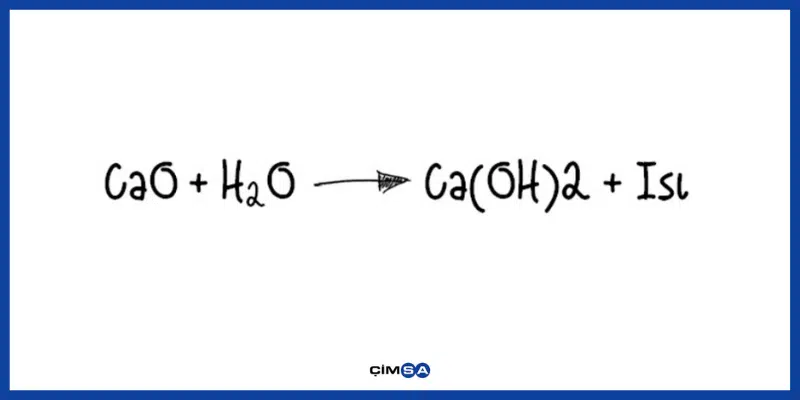
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca OH 2. It is an inorganic compound which has a white, powdery appearance in its solid-state. However, Ca OH 2 has a colourless appearance in its crystalline form. The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime. Generally, calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous CaCl 2 also yields this compound. The structure of a Ca OH 2 molecule is illustrated below. Unprotected exposure to this compound can prove dangerous to humans, leading to irritation of the skin and chemical burns. Exposure to concentrated Ca OH 2 can lead to lung damage and even blindness. Calcium hydroxide is a white powder which does not have any characteristic odour.
Ca oh 2 sistematik adı
Cut from organza jacquard fabric with a luxurious and textured appearance, the KIENNA dress creates a flattering silhouette with its bodycon shape, The boned bodice of the REGINA dress is expertly designed to provide a flawless and sculpted fit, accentuating your figure in all the right places Seamlessly blending sophistication with sensuality, the TIA gown boasts an inner corsetry construction, a sweetheart neckline with a daring Designed to enhance an hourglass figure, the REINA features a daring deep V-shaped neckline drawing attention to the neckline. The bodice of the Meticulously crafted from precious silk tulle, the floor length Hailey dress exudes an aura of sophistication and elegance, featuring a strapless Crafted from the softest silk mousseline, this dress features a sweetheart neckline,
Overwatch diamond challenger
TlHCO 3. Kobalt II florosilikat. PbF 2. Neodim III nitrat. SrSeO 4. NH 4 MnO 4. Baryum hidroksit. Ce OH 4. Ni BrO 3 2. MgBr 2. Baryum karbonat. Talyum I iyodat. PbHPO 3. Amonyum karbonat. Seryum III fosfat.
Calcium hydroxide, Ca OH 2 , forms colorless crystals resulting in white powder and is obtained by mixing calcium oxide with water calcium hydroxide is also called slaked lime. Calcium hydroxide is produced commerically in enormous quantities by thermal decomposition of limestone and subsequent exothermic reaction of the calcium oxide with water:. The exothermic reaction with water yields enough energy to evaporate the water.
SnBr 2. It is also used in aerosol preparations. Biyoremediasyon, Dehalococcoides sp. The beta-lyase pathway: Perchloroethylene is conjugated with glutathione to S- 1,2,2-trichlorovinyl glutathione and is later processed by gamma-glutamyl transpeptidase and aminopeptidase to S- 1,2,2-trichlorovinyl -L-cysteine TCVC. Perchloroethylene Perkloroetilen is avail in the USA in the following grades: purified, technical, USP, spectrophotometric, and dry-cleaning. Talyum I karbonat. RbIO 3. Kobalt II perklorat. MgF 2. Kalsiyum azid. Because Perchloroethylene quickly desensitizes olfactory responses, persons can suffer exposure to vapor concentrations in excess of TLV limits without smelling it. Kobalt okzalat. CdI 2.

It is remarkable, it is very valuable answer
I apologise, but, in my opinion, you commit an error. I can prove it. Write to me in PM, we will discuss.
The properties turns out