Ca oh 2 aq
There is no need to include water in the ionization equation, you just need to include the states in your equation:.
The solubility of calcium hydroxide and the aqueous speciation of Ca II in alkaline medium at various temperatures and background electrolyte concentrations were characterized by solubility measurements applying ICP-OES and potentiometric detection methods. The most important implication of this model is that the total concentration of the dissolved calcium II cannot be decreased below ca. This statement was further validated via precipitation titrations. The standard enthalpies and entropies of the reactions were also calculated from temperature-dependent solubility measurements. Kutus, A.
Ca oh 2 aq
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca OH 2. It is an inorganic compound which has a white, powdery appearance in its solid-state. However, Ca OH 2 has a colourless appearance in its crystalline form. The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime. Generally, calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous CaCl 2 also yields this compound. The structure of a Ca OH 2 molecule is illustrated below. Unprotected exposure to this compound can prove dangerous to humans, leading to irritation of the skin and chemical burns. Exposure to concentrated Ca OH 2 can lead to lung damage and even blindness. Calcium hydroxide is a white powder which does not have any characteristic odour. It is used in industrial settings such as the treatment of waste, the manufacturing of paper, building and processing of food. There are also several medical and dental applications of this compound. For instance, fillings used in root canal treatments often contain calcium hydroxide. Calcium hydroxide, also known as slaked lime with the chemical formula Ca OH 2 is a source of hydroxide ions when dissolved in aqueous solutions.
Download as PDF Printable version.
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide.
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca OH 2. It is an inorganic compound which has a white, powdery appearance in its solid-state. However, Ca OH 2 has a colourless appearance in its crystalline form. The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime. Generally, calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous CaCl 2 also yields this compound. The structure of a Ca OH 2 molecule is illustrated below. Unprotected exposure to this compound can prove dangerous to humans, leading to irritation of the skin and chemical burns. Exposure to concentrated Ca OH 2 can lead to lung damage and even blindness. Calcium hydroxide is a white powder which does not have any characteristic odour.
Ca oh 2 aq
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide. Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0. With a solubility product K sp of 5.
Game of thrones izle 7 sezon 1 bölüm
CAS Number. This statement was further validated via precipitation titrations. View Result. Inorganic compound of formula Ca OH 2. Zr OH 4. Because of its low toxicity and the mildness of its basic properties, slaked lime is widely used in the food industry :. Ni OH 2. Please wait while we load your content Article type Paper. Ho OH 3. Try again? At high pH values due to a common-ion effect with the hydroxide anion , its solubility drastically decreases. It is used in industrial settings such as the treatment of waste, the manufacturing of paper, building and processing of food.
For single-replacement and double-replacement reactions, many of the reactions included ionic compounds—compounds between metals and nonmetals, or compounds that contained recognizable polyatomic ions. Now, we take a closer look at reactions that include ionic compounds. One important aspect about ionic compounds that differs from molecular compounds has to do with dissolution in a liquid, such as water.
Interactive image Interactive image. It has also been used by some indigenous American tribes as an ingredient in yopo , a psychedelic snuff prepared from the beans of some Anadenanthera species. Agriculture Marketing Services. The trees are sprayed when they are dormant in winter to prevent toxic burns from the highly reactive calcium hydroxide. Molecular Orbital Theory. Retrieved 17 July Please wait while we load your content Submitted 29 Feb Nixtamalization significantly increases the bioavailability of niacin vitamin B3 , and is also considered tastier and easier to digest. Generally, calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. The most important implication of this model is that the total concentration of the dissolved calcium II cannot be decreased below ca.

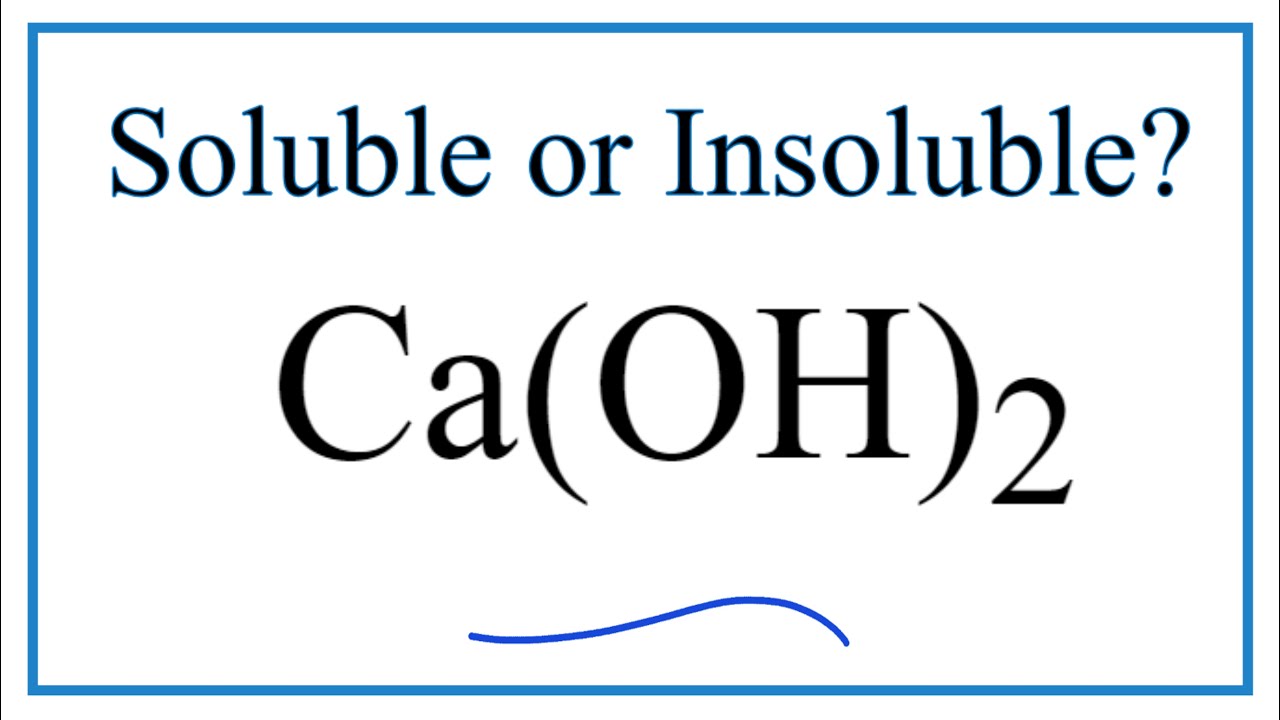
0 thoughts on “Ca oh 2 aq”