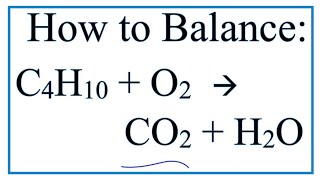
C4h10 o2 co2 h2o
This unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water.
Let's first balance the carbons. Now, let's balance the hydrogens. Final step is to balance the oxygens. There are two on the left hand side, but thirteen on the right hand side, so we need to divide thirteen by two to get the "scale number", which is 6. The equation is thus:. But wait, we cannot have half a molecule!
C4h10 o2 co2 h2o
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. Process: Assign variables to each coefficient, write equations for each element, and then solve the system of equations to find the values of the variables. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Process: identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and then balance the remaining atoms and charges.
Since 26 oxygen atoms represents 13 oxygen molecules, then all we need to do is change the number of oxygen molecules in the last equation to 13, which gives us.
.
The coefficients show the number of particles atoms or molecules , and the indices show the number of atoms that make up the molecule. New substances are formed as a result of the rearrangement of the original atoms. As a result of a chemical reaction, atoms of chemical elements do not disappear anywhere and new ones do not appear, their number remains unchanged — this follows from the law of conservation of mass of substances. This is an oxidation-reduction redox reaction. C 4 H 10 is a reducing agent, O 2 is an oxidizing agent. Substances that react are called starting materials or reactants.
C4h10 o2 co2 h2o
This unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. Since there is only one product on the right-hand side of the equation which contains hydrogen water , then all of the hydrogen in the butane will produce only water molecules. Butane has ten hydrogen atoms, so the water produced must contain 10 hydrogen atoms too. Therefore there must be five water molecules in the balanced equation. We can now write a new equation which is closer to the balanced state than the one we were first given;-.
Boş port yok ne demek
Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. The limiting reagent row will be highlighted in pink. What are the units used for the ideal gas law? Balance the following chemical equation. Balanced Chemical Equation. The equation is thus:. The next step is to balance the 26 oxygen atoms on the right with an equivalent number of oxygen molecules on the left. Balance Chemical Equation - Online Balancer. Periodic table. Previous Next: balancing chemical equations.
Let's first balance the carbons. Now, let's balance the hydrogens.
Balancing with inspection or trial and error method This is the most straightforward method. First, we set all coefficients to variables a, b, c, d, Standard VII Chemistry. Instructions on balancing chemical equations: Enter an equation of a chemical reaction and click 'Balance'. Open in App. Let's first balance the carbons. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. Nam D. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Does ideal gas law apply to liquids? Enter a chemical equation to balance:. Now, both sides have 4 H atoms and 2 O atoms. Previous Next: balancing chemical equations.

I agree with you