Bond order of n2o
The differences here include the number of oxygens attached to nitrogen. Notice the bonding patterns:. Based on the "N"-"O" bond distances of
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties.
Bond order of n2o
.
Equatorial and Axial Positions. Strong Titrate-Strong Titrant Curves.
.
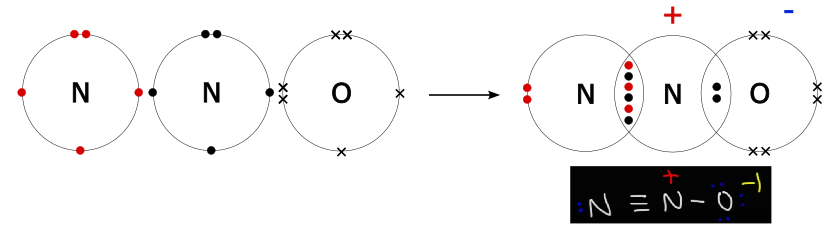
N 2 O nitrous oxide has two nitrogen atoms and one oxygen atom. In the N 2 O Lewis structure, there is a triple bond between two nitrogen atoms, and a single bond between nitrogen and oxygen atom. The left nitrogen atom with a triple bond has one lone pair, and the oxygen atom with a single bond has three lone pairs. In the periodic table , nitrogen lies in group 15, and oxygen lies in group Hence, nitrogen has five valence electrons and oxygen has six valence electrons. Learn how to find: Nitrogen valence electrons and Oxygen valence electrons. We have a total of 16 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here, we have a total of 8 electron pairs. And two bonds are already marked.
Bond order of n2o
The molecular orbital theory has never been so clear as with our bond order calculator. In the following article, we will explain what bond order is, and how to use the bond order formula. You will also find out the difference between bonding and antibonding electrons. Sounds interesting? Let's dive in! If you're reading this, you probably already know the structure of an atom. If you don't, before you read on, check out our atom calculator - it's an excellent introduction to the topic. The bond's order determines the stability of a molecule or ion. The higher the bond order, the stronger is the bond, thus the higher the bond energy.
Is ancestors the humankind odyssey multiplayer
Intro to Electrochemical Cells. General Chemistry Notice the bonding patterns:. Electron Configurations of Transition Metals: Exceptions. Gas Evolution Equations. Magnetic Properties of Complex Ions. Dipole Moment. Solutions: Mass Percent. Wavelength and Frequency. Velocity Distributions. Rate of Radioactive Decay. Related questions What is the Lennard-Jones potential? Intro to General Chemistry 3h 53m. Intro to Radioactivity. Band of Stability: Overview.
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations.
Cell Potential: Standard. Hydrogenation Reactions. Impact of this question views around the world. Precipitation: Ksp vs Q. Intro to Radioactivity. Periodic Table Charges Review. Hess's Law. Why do elements share electrons? Millikan Oil Drop Experiment. Introduction to Organic Chemistry. Extensive Properties. Maxwell-Boltzmann Distribution. Band of Stability: Overview. Significant Figures: Precision in Measurements.

0 thoughts on “Bond order of n2o”