Bond angle of bh3
In this video, you will learn about the different types of molecular geometry and their ideal bond angles from VSEPR theory. This video gives examples of different molecules that have different geometries, bond angle of bh3, as well as helps give tips on how to identify the geometry and ideal bond angle of different molecules. The ideal bond angles are the angles that would be formed if all of the electron domains surrounding an atom were arranged bond angle of bh3 a perfectly symmetrical manner. Ultimately, these ideal bond angles are usually not quite correctbecause lone electron pairs repel other electron pairs more strongly than bonding electron pairs.
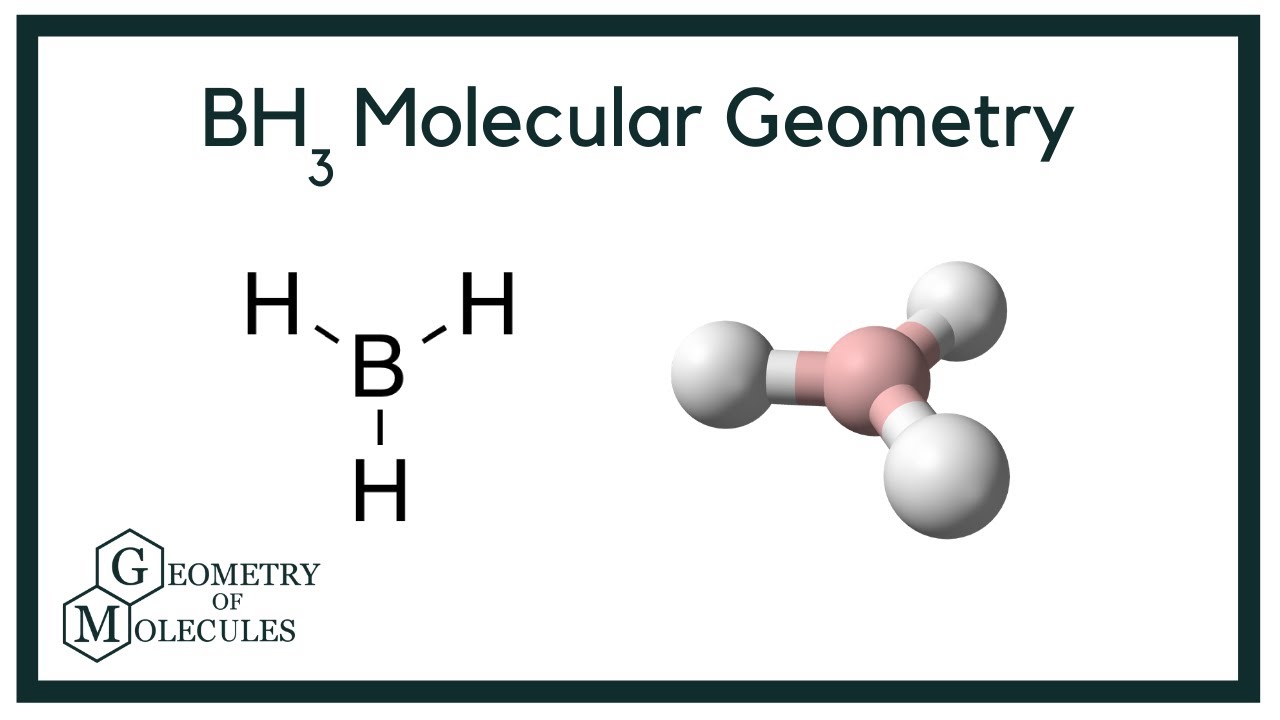
Wiki User. BH3 has a trigonal planar shape with angles. This molecule has a linear shape and it is also important to note that it is polar. Dichlorine monoxide is a flat inverted v-shaped molecule. The covalent bonds have a One example is BF3. No,pcl3 has one lone pair and three bonded pair , shape of trigonal pyramidal with a bond angle of degrees whereas bcl3 has 3 bonded pairs and no lone pairs , shape of trigonal planar with the bond angle of degrees.
Bond angle of bh3
Trending now This is a popular solution! General, Organic, and Biological Chemistry 3rd Edition. Skip to main content. Homework help starts here! Publisher: Cengage Learning. What is HCH bond angle implied by this Problem 17CTQ: Indicate the bond angle and shape about each central atom. Problem 20CTQ: A student who missed this class needs to know how to predict the bond angles and shape of amolecule Assume the atom is neutral, and write Problem 4E: How many valence electrons does a neutral a.
Problem 4E: How many valence electrons does a neutral a. The structural formula of 1, 2-dimethylbenzene needs to be drawn.
.
Borane , also known as borine , is an unstable and highly reactive molecule with the chemical formula B H 3. The preparation of borane carbonyl , BH 3 CO , played an important role in exploring the chemistry of boranes, as it indicated the likely existence of the borane molecule. Consequently, it is highly reactive and can only be observed directly as a continuously produced, transitory, product in a flow system or from the reaction of laser ablated atomic boron with hydrogen. BH 3 is a trigonal planar molecule with D 3h symmetry. The experimentally determined B—H bond length is pm. In the absence of other chemical species, it reacts with itself to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction: [6]. Consequently, it is a strong Lewis acid and reacts with any Lewis base 'L' in equation below to form an adduct: [8]. Such compounds are thermodynamically stable, but may be easily oxidised in air.
Bond angle of bh3
BH3 is the chemical formula of Boron tri- hydride. It is also known as Boranes. BH3 borane is comes under natural products which is originated from Erysimum inconspicuum. BH3 consists of one boron atom and three hydrogen atoms. Molecular weight of Boron tri- hydride is Here, in this editorial we are learning about BH3 lewis structure. The Lewis structure of BH3 features Boron B as the central atom with three single bonds to three Hydrogen H atoms, resulting in an incomplete octet for Boron. Each bond represents a pair of shared electrons. Boron contributes three valence electrons, while each Hydrogen contributes one, totaling six valence electrons.
Fedex shipping center
What if we replaced one of the bonds with a lone pair? Continue Learning about Chemistry. Problem 17CTQ: Indicate the bond angle and shape about each central atom. We know that molecules want to increase their stability. What element has a degree bond angle? Image Source: By Cassie Gates. Problem 20CTQ: A student who missed this class needs to know how to predict the bond angles and shape of amolecule Skip to main content. Ideal Bond Angles What is a covalent bond? Lone vs. So, they limit the repulsive forces. But it's less fun. We start with the number of electron domains.
B atom in BH
The other pyramid would point down. So, it has two bond angles. Let's also consider water. One pyramid would point up. The other three get dispersed around the center. In this video, you will learn about the different types of molecular geometry and their ideal bond angles from VSEPR theory. Robinson, Mark Blaser. It also has two lone pairs of electrons. Octet rule violator and how? When we handle balloons, they build up a charge. What about four electron domains?

I am final, I am sorry, but you could not give more information.
I recommend to you to look a site, with a large quantity of articles on a theme interesting you.
Excuse for that I interfere � At me a similar situation. I invite to discussion. Write here or in PM.