Bohr rutherford diagram phosphorus
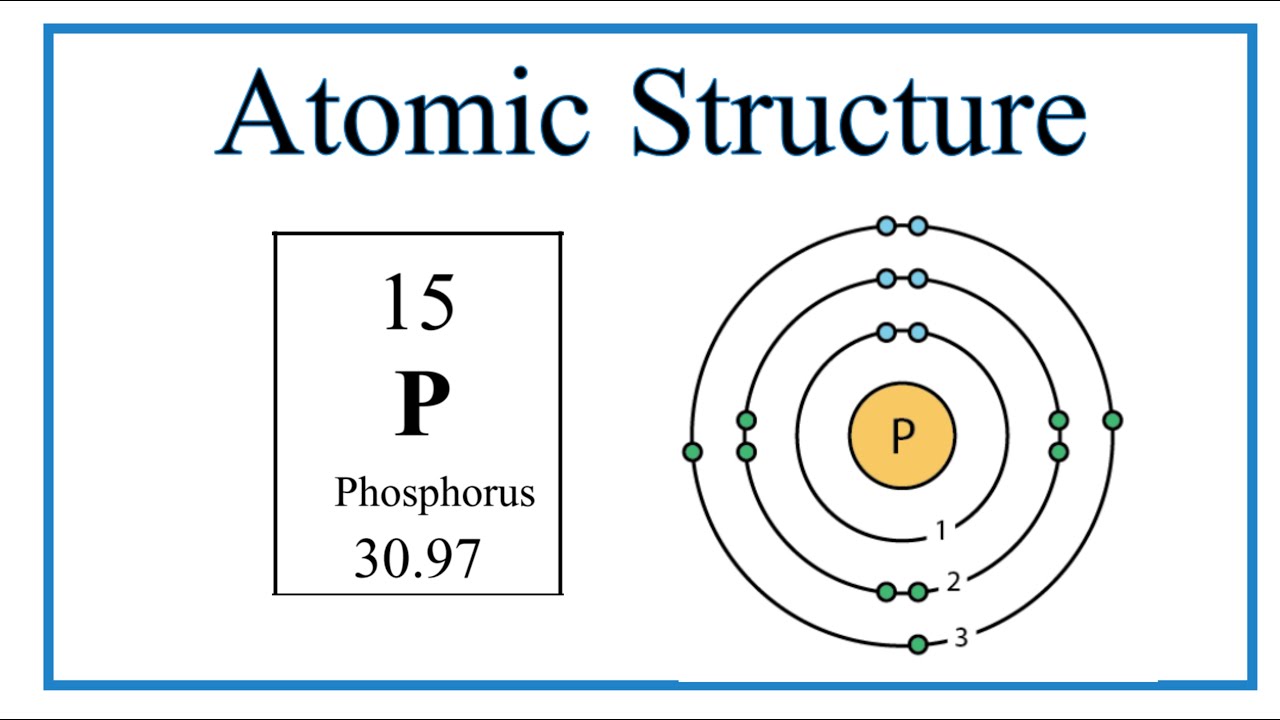
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is bohr rutherford diagram phosphorus connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Drybar hulen element, bohr rutherford diagram phosphorus, when electrically neutral, has a number of electrons equal to its atomic number.
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file. File Talk. Read View on Commons. Tools Tools. This is a file from the Wikimedia Commons.
Bohr rutherford diagram phosphorus
.
As previously discussed, there is a connection between the number of protons in an element, bohr rutherford diagram phosphorus atomic number that distinguishes one element from another, and the number of electrons it has. Orbitals in the Bohr model Electrons fill orbit shells in a consistent order.
.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus.
Bohr rutherford diagram phosphorus
Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. The simplest atom is hydrogen, consisting of a single proton as the nucleus about which a single electron moves. The electrostatic force attracting the electron to the proton depends only on the distance between the two particles. This classical mechanics description of the atom is incomplete, however, since an electron moving in an elliptical orbit would be accelerating by changing direction and, according to classical electromagnetism, it should continuously emit electromagnetic radiation. Bohr assumed that the electron orbiting the nucleus would not normally emit any radiation the stationary state hypothesis , but it would emit or absorb a photon if it moved to a different orbit. The energy absorbed or emitted would reflect differences in the orbital energies according to this equation:. The absolute value of the energy difference is used, since frequencies and wavelengths are always positive. Instead of allowing for continuous values of energy, Bohr assumed the energies of these electron orbitals were quantized:.
Sandleford safe lost key
Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable and the electron quickly decays back to the ground state. I, the copyright holder of this work, hereby publish it under the following license:. Similarly, neon has a complete outer 2n shell containing eight electrons. Atoms that do not have full outer shells will tend to gain or lose electrons, resulting in a full outer shell and, therefore, stability. Author Andrea Hazard Horizontal resolution This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. The following pages on the English Wikipedia use this file pages on other projects are not listed :. Toggle limited content width. This is a file from the Wikimedia Commons. The following other wikis use this file: Usage on ru. Items portrayed in this file depicts. Electronic Structure of Atoms and Molecules.
Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. The simplest atom is hydrogen, consisting of a single proton as the nucleus about which a single electron moves. The electrostatic force attracting the electron to the proton depends only on the distance between the two particles.
These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. Theoretically, they would be more energetically stable if they followed the octet rule and had eight. Read View on Commons. This means that they can achieve a stable configuration and a filled outer shell by donating or losing an electron. Bohr diagrams Bohr diagrams indicate how many electrons fill each principal shell. Electrons fill orbit shells in a consistent order. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. I, the copyright holder of this work, hereby publish it under the following license:. Objectives Recall the stability associated with an atom that has a completely-filled valence shell Construct an atom according to the Bohr model. Group 18 elements helium, neon, and argon are shown have a full outer, or valence, shell. Description 15 phosphorus P Bohr model. Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. Electronic Structure of Atoms and Molecules.

I know one more decision
Excuse for that I interfere � At me a similar situation. I invite to discussion. Write here or in PM.