Bohr diagram for lithium
Lithium is the group 1 chemical element. It has atomic number 3 and is denoted by the symbol Li.
Top page correct Bohr model including the two-electron atoms. Lamb shift is an illusion! Our new Bohr model has suceeded in calculating the Helium ionization energy more correctly than the quantum mechanical variational methods as shown in the Top page. Lithium belongs to the alkali metal group of chemical elements, and has the atomic number 3. It is the lightest metal, and highly reactive and flammable though more stable than the other alkali metals. Naturally occurring lithium is composed of two stable isotopes, Li6 and Li7, the latter being the more abundant
Bohr diagram for lithium
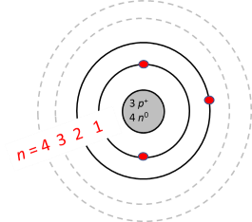
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr — These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. These energy levels are designated by a number and the symbol "n. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. Energy from a photon of light can bump it up to a higher energy shell, but this situation is unstable and the electron quickly decays back to the ground state. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
January 19, The electron that absorbs energy jumps upwards while the electron that loses energy jumps downward. There are 2 electrons present in the K shell and 1 electron in the L shell.
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file. File Talk. Read View on Commons. Tools Tools. This is a file from the Wikimedia Commons.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number. An early model of the atom was developed in by Danish scientist Niels Bohr —
Bohr diagram for lithium
Lithium is the group 1 chemical element. It has atomic number 3 and is denoted by the symbol Li. It is a highly reactive and flammable substance due to which it is usually stored under inert conditions, mostly immersed in an inert liquid, like kerosene. It is an alkali metal that exhibits metallic luster and is prone to corrosion when exposed to moisture. It usually occurs as an ionic compound such as pegmatitic minerals.
Liberty flights vape
The atomic symbol of the element represents the nucleus while the dots represent the electrons. Electron Shells Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. Creative Commons Attribution-ShareAlike 4. Is it like helium atom He? The 3 electrons revolve around the nucleus. Let us begin by drawing the nucleus for the Lithium atom, for which we will have to calculate the number of protons and neutrons in the Lithium atom. Hydrogen is excluded because it can hold a maximum of 2 electrons in its valence shell. When the electron 1 has moved one quarter of its orbit and its x-coordinate is zero, this program checked the y-component of the electron 1 velocity last VY. From the discussion in the previous section, we know that the Lithium atom consists of 3 valence electrons. I, the copyright holder of this work, hereby publish it under the following licenses:. I, the copyright holder of this work, hereby publish it under the following license:. In this model, the electron 1 moves on the X-Y plane, the electron 2 moves on the X-Z plane. This small error is probably caused by using the "approximate" orbit of the 2S electron. The electrons are also capable of jumping up and down from their orbits into the higher or lower orbit, respectively. See the top page in detail.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos.
Next we try the litium atom Li. Creative Commons Attribution-ShareAlike 4. It is a highly reactive and flammable substance due to which it is usually stored under inert conditions, mostly immersed in an inert liquid, like kerosene. Upload file Recent changes Latest files Random file Contact us. Electronic Structure of Atoms and Molecules. Lamb shift is an illusion! As shown, helium has a complete outer electron shell, with two electrons filling its first and only shell. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. In comparison, the group 1 elements, including hydrogen H , lithium Li , and sodium Na , all have one electron in their outermost shells. If we consider all components of the Coulomb force against the electrons, the electron's motion becomes as shown in Fig.

Completely I share your opinion. In it something is also idea good, I support.
Rather useful piece
Has casually come on a forum and has seen this theme. I can help you council. Together we can find the decision.