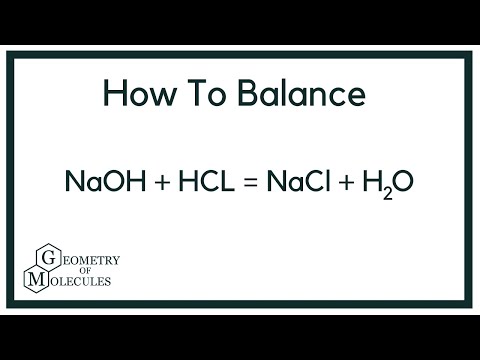
Balanced equation for hydrochloric acid and sodium hydroxide
Skip to main content.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction.
Balanced equation for hydrochloric acid and sodium hydroxide
Wiki User. Hydrochloric Acid would be the stronger acid, as Sodium Hydroxide is an alkali. Since Sodium Hydroxide is a base and hydrochloric acid is an acid, you will make water and sodium chloride. Sodium hydroxide is a base and hydrochloric acid is an acid. Both are not same. Can you store 6. When hydrochloric acid is neutralized by sodium hydroxide, the salt formed is sodium chloride NaCl. If Sodium hydroxide and Hydrochloric acid are combined they will react and produce water and Sodium chloride. Tags Acids and Bases Subjects. Log in. Study now See answer 1. Best Answer. Sodium hydroxide plus hydrochloric acid equals sodium chloride plus water.
Power and Root Functions.
.
Acids and bases have another property: they react with each other to make water and an ionic compound called a salt. A salt , in chemistry, is any ionic compound made by combining an acid with a base. A reaction between an acid and a base is called a neutralization reaction and can be represented as:. Neutralization reactions are an example of irreversible double-replacement reactions, like the ones we studied in CHE What is the name of the salt that is formed? The balanced chemical reaction is as follows:. In CHE , we examined how ionic compounds dissociate in water. In this chapter, we also learned that acids can also undergo dissociation in water.
Balanced equation for hydrochloric acid and sodium hydroxide
In association with Nuffield Foundation. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. You have to decide if this experiment is suitable to use with different classes, and look at the need for preliminary training in using techniques involved in titration see Teaching notes. What follows here assumes that teachers have judged the class to be capable of doing this experiment using a burette with reasonable expectation of success. Assuming that the students have been given training, the practical work should, if possible, start with the apparatus ready at each work place in the laboratory.
Chick fil a muskogee
Measuring Radioactivity. Quantum Numbers: Spin Quantum Number. Lewis Dot Structures: Exceptions. Sodium hydroxide is a base and hydrochloric acid is an acid. Electron Geometry. Verified Solution. Cell Notation. Phase Diagrams. Chemical Kinetics 2h 42m. Intro to Redox Reactions. Ester Reactions: Saponification. Best for: complex redox reactions, especially in acidic or basic solutions. Naming Molecular Compounds.
Pouring concrete and working it are messy jobs. In the process, a lot of wastewater with an alkaline pH is generated.
Hydrogen Compounds. Neutron to Proton Ratio. Atomic, Ionic, and Molecular Solids. Diprotic Acids and Bases. This method uses algebraic equations to find the correct coefficients. Best Answer. Hess's Law. Test for Ions and Gases. Metric Prefixes. Periodic Trend: Metallic Character. Orientations of D Orbitals. Diprotic Acids and Bases Calculations.

It seems brilliant phrase to me is