B lewis structure
Together the three resonance structures suggest partial double-bond character in the Be-X bond, which results in an intermediate bond length between a single and double bond.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:. Lewis Structures We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions.
B lewis structure
Dec 15, Chemistry. This structure is used to predict the chemical behavior of atoms and molecules. But how to draw the lewis dot structure? Lewis dot structure. Boron belongs to group 13 of the periodic table, and its electronic configuration is 2,3. This means it has three electrons in its valence shell, two in the 2s orbital and one in the 2p orbital. A Lewis dot structure is a graphic representation of the electron distribution around atoms. This also predicts the type and number of bonds formed around an atom. It is also possible to predict the geometry of a molecule by using a lewis dot structure. Here are some steps on how to draw lewis dot diagram of a moleculeto follow while drawing a lewis dot structure of any molecule:. Sum up the valence electrons in the molecule from all the atoms. Covalent bonds form when an electron from every atom forms an electron pair. Step 2 indicates the number of required electrons, while Step 1 indicates the number of electrons you have.
To complete the octets of electrons, choose the central b lewis structure before placing electrons around them. To determine electronegativity, use periodic table trends or a table that records electronegativity values. Both of these gases also cause problems: CO is toxic and CO 2 has been implicated in global climate change.
A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron configuration, i. Lewis structure does NOT attempt to explain the geometry of molecules, how the bonds form, or how the electrons are shared between the atoms.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven.
B lewis structure
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons as shown in Figure Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:.
Zoo cartoon images
Of the 24 valence electrons available in NO 3 - , 6 were used to make the skeletal structure. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Key Takeaways Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Check Your Learning What is the Lewis electron dot symbol for each element? Generally, the least electronegative element should be placed in the center. Skip to main content. Exceptions to the octet rule occur for odd-electron molecules free radicals , electron-deficient molecules, and hypervalent molecules. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The following is an example of how to draw the "best" Lewis structure for NO 3 - learning by example. This type of molecule, called a fullerene, shows promise in a variety of applications.
A Lewis structure shows the bonding between atoms as short lines some books use pairs of dots and non-bonding valence electrons as dots. To learn about Lewis structures, we will start with the Lewis symbol. The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots.
Lewis Structures We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions. Make double bonds to lone pairs on outside atoms if any bonds remain from Step 3. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Read More. Tarr , Inorganic Chemistry 2nd ed. Chemical structures may be written in more compact forms, particularly when showing organic molecules. Non-structural formulas Empirical formula Molecular formula. Two solid lines between two atoms represent a double bond. Now we need to add lone pairs of electrons. You should learn to recognize any of the possible Lewis structures.

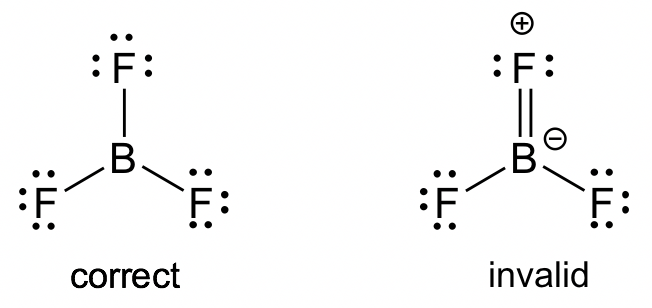
I consider, that you commit an error. I suggest it to discuss. Write to me in PM, we will communicate.