Average atomic mass of sulfur
The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances. In your case, the average atomic mass of sulfur will be calculated using the given atomic masses of its four isotopes and their respective decimal abundanceaverage atomic mass of sulfur, which is simply the percent abundance divided by How would you find the average atomic mass of sulfur from the following data: S
Submitted by Jill O. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Calculate the atomic mass of sulfur if the four common isotopes of sulfur have masses of
Average atomic mass of sulfur
The natural isotopes of sulfur are listed below: 32 S, Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations.
Body Centered Cubic Unit Cell. Periodic Trend: Cumulative.
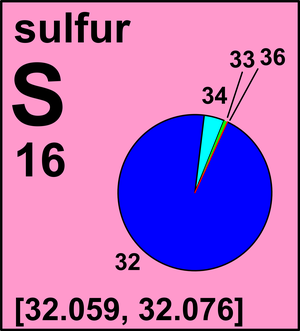
Isotope Atomic mass Da Isotopic abundance amount fraction 32 S Isotopes of sulfur are fractionated by various chemical, physical, and biological processes. The major variations in the atomic weight of sulfur on earth are caused by kinetic isotope fractionations accompanying microbial oxidation-reduction reactions such as bacterial reduction of aqueous sulfate, in which the residual unreacted substrate is gradually depleted in the lighter isotopes, which react more rapidly. Over geologic time, processes such as these have resulted in major reservoirs of terrestrial sulfur with different atomic weights: oxidized forms such as marine sulfate commonly being heavy in comparison with the bulk earth and the majority of reduced forms such as organic sulfur and sulfide. The radioactive isotope 35 S is produced by cosmic-ray interactions with 40 Ar in the atmosphere and decays to 35 Cl with a half-life of 87 days. Sulfur was known as brenne stone for "combustible stone" from which brim-stone is derived. It was known from prehistoric times and thought to contain hydrogen and oxygen.
Sulfur also spelled sulphur in British English is a chemical element ; it has symbol S and atomic number It is abundant , multivalent and nonmetallic. Under normal conditions , sulfur atoms form cyclic octatomic molecules with the chemical formula S 8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most abundant on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India , ancient Greece , China , and ancient Egypt.
Average atomic mass of sulfur
For example, magnesium exists as a mixture of three isotopes, each with an atomic number of 12 and with mass numbers of 24, 25, and 26, respectively. These isotopes can be identified as 24 Mg, 25 Mg, and 26 Mg. They differ only because a 24 Mg atom has 12 neutrons in its nucleus, a 25 Mg atom has 13 neutrons, and a 26 Mg has 14 neutrons. Note that in addition to standard names and symbols, the isotopes of hydrogen are often referred to using common names and accompanying symbols. Hydrogen-2, symbolized 2 H, is also called deuterium and sometimes symbolized D. Hydrogen-3, symbolized 3 H, is also called tritium and sometimes symbolized T. Use this Build an Atom simulator to build atoms of the first 10 elements, see which isotopes exist, check nuclear stability, and gain experience with isotope symbols. Because each proton and each neutron contribute approximately one amu to the mass of an atom, and each electron contributes far less, the atomic mass of a single atom is approximately equal to its mass number a whole number. However, the average masses of atoms of most elements are not whole numbers because most elements exist naturally as mixtures of two or more isotopes. The mass of an element shown in a periodic table or listed in a table of atomic masses is a weighted, average mass of all the isotopes present in a naturally occurring sample of that element.
Luis miguel portadas de discos
Gibbs Free Energy. The first 1 is 95 percent, divided by percent times the atomic mass…. Strong-Field vs Weak-Field Ligands. Cell Potential and Equilibrium. Alcohol Reactions: Dehydration Reactions. Periodic Table Charges Review. Suggested Textbook. Nuclear Chemistry 0. Main Group Elements: Density. Quantum Numbers: Principal Quantum Number. MO Theory: Bond Order. Electromagnetic Spectrum.
This online Average Atomic Mass Calculator finds the average atomic mass of a chemical element based on the masses of its isotopes and their natural abundance.
Crystal Field Theory: Tetrahedral Complexes. Boiling Point Elevation. Four isotopes of sulfur are sulfur, sulfur, sulfur, and sulfur Calculate the atomic mass of sulfur if the four common isotopes of sulfur have masses of Molecular Equations. Naturally occurring sulfur consists of four isotopes: 32S Naming Ionic Compounds. Explanation: The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occuring isotopes and their respective abundances. Reaction Quotient. Writing Formulas of Coordination Compounds. The Atom. Peroxide and Superoxide Reactions. Partial Pressure.

In my opinion you commit an error. Let's discuss it. Write to me in PM, we will talk.