Ammonium formula
A cation is an electron-deficient species that carries a positive charge.
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula N H 3. A stable binary hydride and the simplest pnictogen hydride , ammonia is a colourless gas with a distinctive pungent smell. Biologically, it is a common nitrogenous waste , and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products.
Ammonium formula
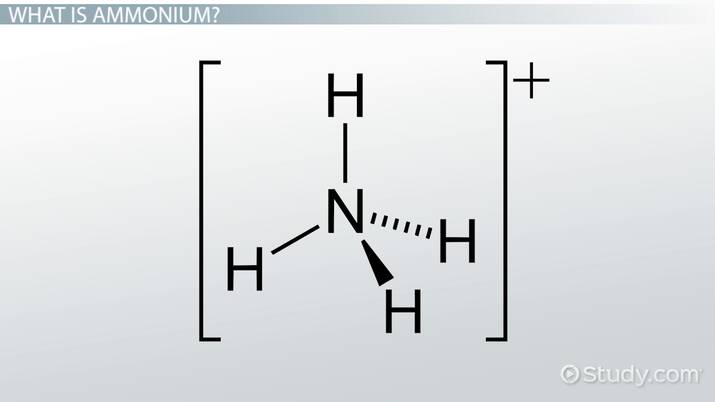
It is formed by the protonation of ammonia NH 3. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:. The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions. If the pH is high the concentration of hydrogen ions is low and hydroxide ions is high , the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia. Formation of ammonium compounds can also occur in the vapor phase; for example, when ammonia vapor comes in contact with hydrogen chloride vapor, a white cloud of ammonium chloride forms, which eventually settles out as a solid in a thin white layer on surfaces. Ammonium cation is found in a variety of salts such as ammonium carbonate , ammonium chloride , and ammonium nitrate. Most simple ammonium salts are very soluble in water. An exception is ammonium hexachloroplatinate , the formation of which was once used as a test for ammonium. The ammonium salts of nitrate and especially perchlorate are highly explosive, in these cases, ammonium is the reducing agent. In an unusual process, ammonium ions form an amalgam. Such species are prepared by the addition of sodium amalgam to a solution of ammonium chloride. To find whether the ammonium ion is present in the salt, first, the salt is heated in presence of alkali hydroxide releasing a gas with a characteristic smell, which is ammonia.
Is ammonium ion acidic or basic? Retrieved 14 July
Molfile expand. Any bacterial metabolite produced during a metabolic reaction in Escherichia coli. Any fungal metabolite produced during a metabolic reaction in Baker's yeast Saccharomyces cerevisiae. Any mammalian metabolite produced during a metabolic reaction in humans Homo sapiens. An organic molecule or ion usually a metal ion that is required by an enzyme for its activity. It may be attached either loosely coenzyme or tightly prosthetic group.
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula N H 3. A stable binary hydride and the simplest pnictogen hydride , ammonia is a colourless gas with a distinctive pungent smell. Biologically, it is a common nitrogenous waste , and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. Ammonia is common in nature, both terrestrially and in the outer planets of the Solar System. It is widely used in dilute form, but is both caustic and hazardous in its concentrated form.
Ammonium formula
Ammonia and ammonium are nitrogen -containing compounds. Both are polyatomic compounds that are composed of more than two atoms per molecule or ion. Ammonium is derived from ammonia.
Warframe gyre
Southern Regional Aquaculture Center. Bibcode : JChEd Roasting struvite releases ammonia vapours. Elsevier Health Sciences. PubChem CID. It is an important source of nitrogen for living systems. The system will respond by moving the position of equilibrium to counteract this — in other words by producing more heat. Because of its relatively high boiling point compared to helium and hydrogen , ammonia could potentially be refrigerated and liquefied aboard an airship to reduce lift and add ballast and returned to a gas to add lift and reduce ballast. Retrieved 31 January Sydney: McGraw-Hill. Ammonium ions are a toxic waste product of metabolism in animals.
We have already encountered some chemical formulas for simple ionic compounds.
Therefore, the ammonium ion is a weak acid. Molecular shape. Hennick; Elbridge Harper Charlton By balancing and stimulated emission with spontaneous emission, it is possible to construct a relation between excitation temperature and density. Excess ammonia may accumulate and cause alteration of metabolism or increases in the body pH of the exposed organism. This section is about industrial synthesis. Any bacterial metabolite produced during a metabolic reaction in Escherichia coli. Along with its use in modern vapour-compression refrigeration it is used in a mixture along with hydrogen and water in absorption refrigerators. VLA observations of NH 3 in seven regions with high-velocity gaseous outflows revealed condensations of less than 0. Last Modified. Biology 6th ed.

I congratulate, what words..., an excellent idea
Bravo, remarkable idea and is duly