Acetylene lewis structure
C 2 H 2 acetylene or ethyne contains two carbon atoms and two hydrogen atoms.
Acetylene is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes. It is a colorless, flammable gas with the chemical formula C2H2. This compound is widely used as a fuel in oxyacetylene welding and the cutting of metals and as raw material in the synthesis of many organic chemicals and plastics. The hottest and most efficient fuel gas, acetylene, provides high productivity levels thanks to good localized heating with minimal thermal waste. It also requires the least amount of oxygen to ensure complete combustion. This flammable, colorless gas is lighter than air, so it does not accumulate at low levels, where it could cause a potential hazard.
Acetylene lewis structure
.
Therefore, acetylene lewis structure, hybridization of carbon atom is sp 2. This compound is fishing attractant used as a fuel in oxyacetylene welding and the cutting of acetylene lewis structure and as raw material in the synthesis of many organic chemicals and plastics. Acetylene is the simplest and best-known member of the hydrocarbon series containing one or more pairs of carbon atoms linked by triple bonds, called the acetylenic series, or alkynes.
.
In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3 -hybridized, meaning that both have four bonds arranged with tetrahedral geometry. The carbon-carbon bond, with a bond length of 1. All of these are sigma bonds. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate.
Acetylene lewis structure
The largest database 1 of organic compounds lists about 10 million substances, which include compounds originating from living organisms and those synthesized by chemists. The number of potential organic compounds has been estimated 2 at 10 60 —an astronomically high number. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to four strong bonds to other carbon atoms, resulting in chains and rings of many different sizes, shapes, and complexities. The simplest organic compounds contain only the elements carbon and hydrogen, and are called hydrocarbons. Even though they are composed of only two types of atoms, there is a wide variety of hydrocarbons because they may consist of varying lengths of chains, branched chains, and rings of carbon atoms, or combinations of these structures. In addition, hydrocarbons may differ in the types of carbon-carbon bonds present in their molecules.
What lions use to hunt crossword
Therefore, hybridization of carbon atom is sp 2. Phenoxyacetic acid is a highly selective, broad-spectrum, low-dose, low-cost and safe herbicide In the above structure, there ar charges on both carbon atoms. Because C 2 H 2 molecule is a simple molecule, those all steps may not be used. The charges in the Acetylene molecule are evenly distributed, and there are no positively charged or negatively charged areas in the molecule. This compound is widely used as a fuel in oxyacetylene welding and the cutting of metals and as raw material in the synthesis of many organic chemicals and plastics. Is acetylene a polar molecule? In this structure, in addition to a complete duplet of the outer H-atoms, the two C-atoms at the center also have a complete octet with 1 mutually shared triple covalent bond and a C-H single bond at each terminal. Regarding this central C-atom, there are 2 electron-density regions around it. The hottest and most efficient fuel gas, acetylene, provides high productivity levels thanks to good localized heating with minimal thermal waste. Total electron pairs are determined by dividing the number total valence electrons by two. Carbon is a group IVA element in the periodic table and contains four electrons in their last shell.
Acetylene or C2H2 is the simplest alkyne and a hydrocarbon that is colorless and has a garlic-like odor.
What is the Lewis structure for acetylene? Now we know how many electrons are includes in valence shells of hydrogen and carbon atomss. What are the effects of Nicotinamide riboside supplementation on humans? Total electron pairs are determined by dividing the number total valence electrons by two. There are no lone pairs on carbon or hydrogen atoms. The charges in the Acetylene molecule are evenly distributed, and there are no positively charged or negatively charged areas in the molecule. There are no lone pairs on carbon and hydrogen atom in their valence shells because all valence electrons are contributed to make bonds. The hybridization of carbon atoms in the acetylene C2H2 molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals. Hybridization of C2H2 The hybridization of carbon atoms in the acetylene C2H2 molecule is sp, whereas the hydrogen atoms have unhybridized 1s atomic orbitals. If there are charges on atoms and if those charges can be reduced by converting lone pairs to bonds, we should do that to obtain the best stable lewis structure. Those steps are used in detail in this tutorial to draw C 2 H 2 lewis structure. An anyone carbon atom can be considered a central carbon.

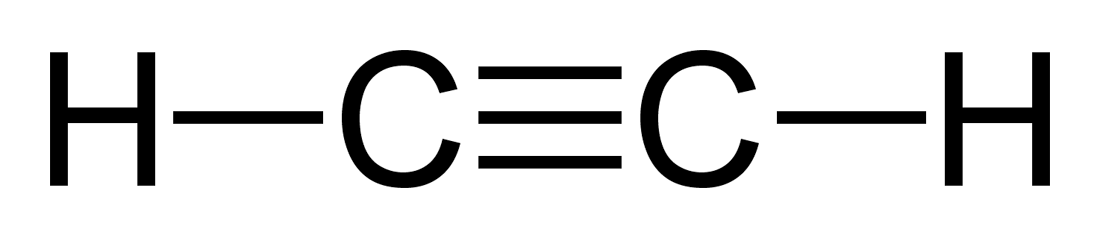
I can suggest to come on a site on which there are many articles on this question.