Acetic acid lewis dot structure
Weekly Web Work 7: a. Submissions are no longer accepted. Please type your last name, first name:. Please type the last five digits of your ID number:.
Lewis Dot Structures. This assignment is past due and can no longer be submitted. The purpose of this assignment is to practice drawing Lewis dot structures. You will need to have a pencil and paper to use while working on this assignment. In order to draw the Lewis structure of acetic acid, you need to determine the number of valence electrons.
Acetic acid lewis dot structure
.
How many valence electrons does chloroform have? Draw a bond between carbon and each surrounding atom.
.
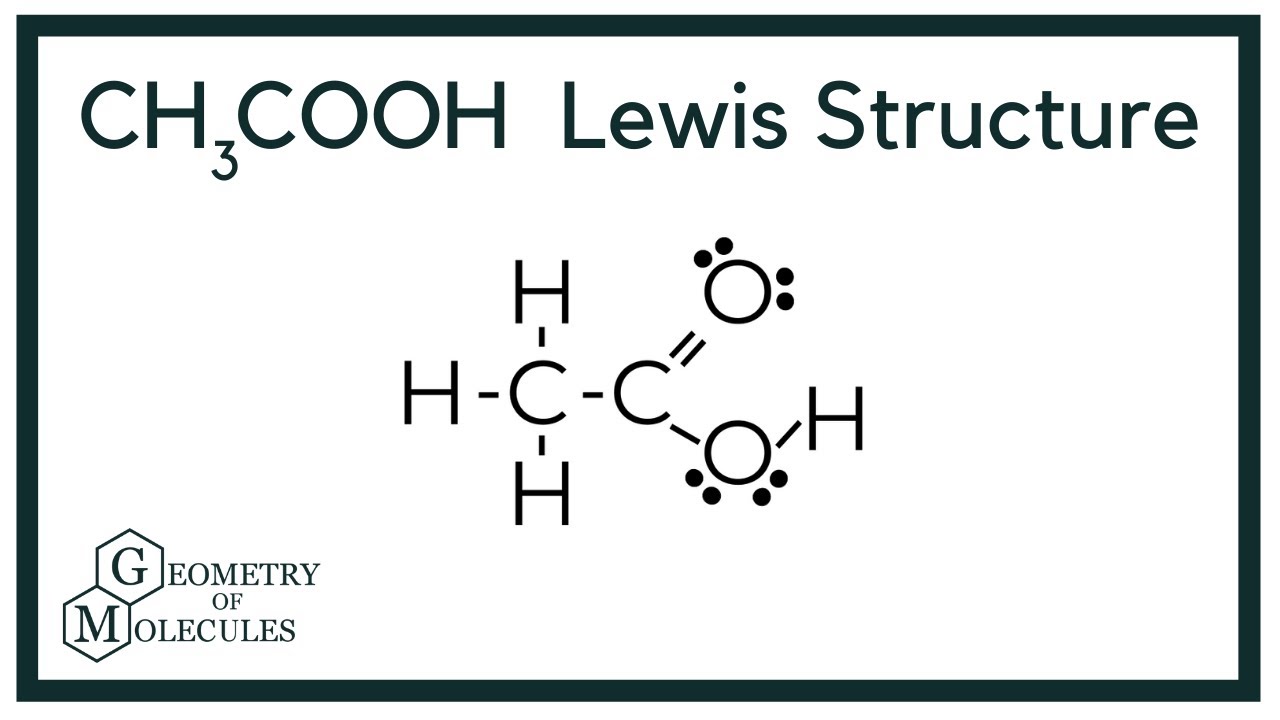
It corrodes both metals and tissues. Long-term acetic acid exposure can cause serious irritation in the eyes, skin, nose, throat, and other body parts, among other things. When acetic acid reaches 40 degrees Celsius, it becomes flammable and explosive. In its liquid state, acetic acid is a polar or protic solvent. Lewis structure , also known as electron dot structure, aids in understanding how atoms or valence electrons are grouped in a molecule. As a result, the first step in constructing a Lewis diagram for every molecule is to figure out how many valence electrons are present.
Acetic acid lewis dot structure
Each carbon atom is also bonded to three hydrogen H atoms, and the remaining oxygen atom has two lone pairs of electrons. Valence electrons are the outermost electrons in an atom that are involved in chemical bonding. To determine the number of valence electrons in hydrogen sulfide , add the number of valence electrons in each atom. Hydrogen has one valence electron, while oxygen has six valence electrons, and carbon has four electrons. The organic molecule is formed by a carbon chain and oxygen are attached to one carbon. The remaining oxygen atoms are bonded 3 to one carbon and one to a oxygen atom. Hydrogen can only form one bond, while carbon can form four bonds, and oxygen up to two bonds. Draw the carbon chain, then put the oxygens and finally the hydrogens.
Redbar comedy club
If yes, cool. In order to draw the Lewis structure of acetic acid, you need to determine the number of valence electrons. Include the number of single, double, or triple bonds and the location of any lone pairs. Look at the Lewis structure of acetic acid. Place lone pairs of electrons around any atom still needing an octet. Now, place hydrogen and chlorine atoms around the carbon. Our Chemical World. The next step is to write carbon as the central atom. Do all of the atoms have an octet or a duet in the case of hydrogen? Draw a bond between carbon and each surrounding atom. If not, you need to add lone pairs to satisfy each atom. Our Chemical World. Why could this be?
In this comprehensive guide, we will take you through the process of drawing the Lewis structure for CH3COOH, also known as acetic acid, a molecule with significant importance in organic chemistry and beyond. Find the Total Valence Electrons. Carbon C contributes 4 valence electrons, hydrogen H has 1 valence electron, and oxygen O contributes 6 valence electrons each.
Remember, 2 electrons are all hydrogen needs to be satisfied. Look at the structure on the left. If not, do what all chemists do and rework your structure. You may change your mind as often as you wish. How many lone pairs of electrons? How many lone pairs of electrons? How many double bonds? Now, place hydrogen and chlorine atoms around the carbon. Do all of the atoms have an octet or a duet in the case of hydrogen? If yes, cool. Please type your last name, first name: Please type the last five digits of your ID number: Please type a nickname: in case your answer gets used in class.

Do not despond! More cheerfully!
I think, to you will help to find the correct decision. Be not afflicted.
It is not logical