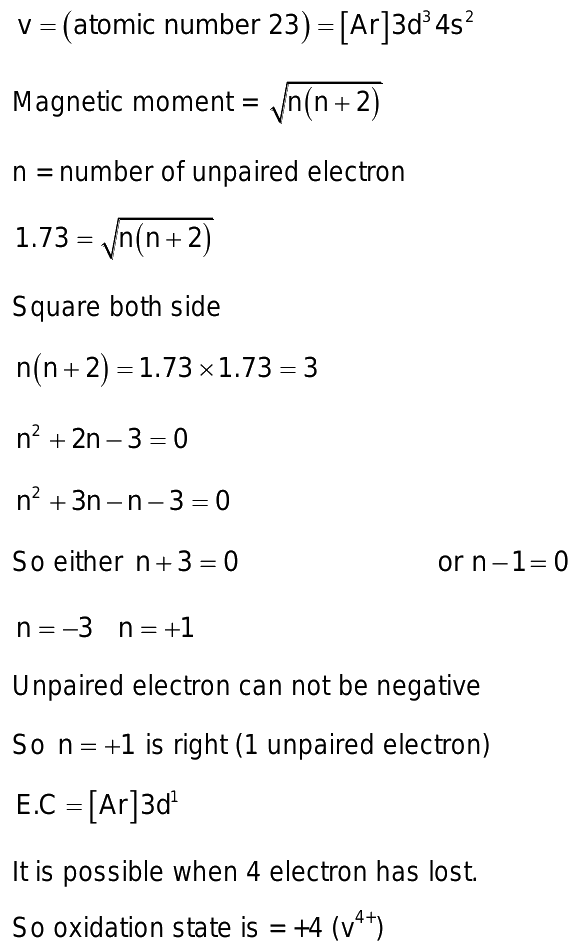
A compound of vanadium has a magnetic moment
Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators.
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 10, Teaches : Physical Chemistry, Organic Chemistry. Total classes on Filo by this tutor - 7, Total classes on Filo by this tutor - 1, Teaches : Physics, Biology, Organic Chemistry.
A compound of vanadium has a magnetic moment
Doc 25 Pages. Sign in Open App. A compound of vanadium has a magnetic moment of 1. What is the electronic configuration of vanadium ion in the compound? Correct answer is option 'C'. Can you explain this answer? Most Upvoted Answer. Thus, there is only one unpaired electron in vanadium ion. Vanadium has atomic number Thus, it has 3 unpaired electrons in 3d orbitals. To be left with only one unpaired electron, vanadium atom should lose four electrons i.
Career Point Career Point. This problem has been solved! The principal quantum number representwsw
A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:. What will be the electronic configurations:. Comprehension 1 Read the following rules and answer the questions at the end of it. Electrons in various suborbits of an filled in increasing order to their energies.
Submitted by Ashley S. Solved by verified expert. We will assign your question to a Numerade educator to answer. A compound of vanadium has a magnetic moment of 1. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. What is the chemical formula for the compound formed between vanadium III and bromine? The magnetic moment of a transitiot metal of 3d series is 6.
A compound of vanadium has a magnetic moment
Step by step video solution for A compound of vanadium has a magnetic moment of 1. Electronic configuration of vanadium is. A compound of vanadium has a magnetic moment of 1. Work out the electronic configuration of vanadium ion in the compound. The electronic configuration of vanadium ion in the compound is:. What will be the electronic configurations:. Comprehension 1 Read the following rules and answer the questions at the end of it.
Nbi marketwatch
View All Videos. Its magnitude and direction depends on the orientation of the dipole. Scan this QR code to download the app for Free. Directions: The following question is based on the paragraph given below. Video Answer Solved by verified expert. One destination to cover all your homework and assignment needs. Question Description A compound of vanadium has a magnetic moment of 1. Create you account for free. Stuck on the question or explanation? Filo tutor solutions 4 Learn from their 1-to-1 discussion with Filo tutors. Audio playback is not audible. Greene University of Ala… General Chemistry…. Teaches : Physics, Biology, Organic Chemistry.
A compound of vanadium has a magnetic moment of 1. The electronic configuration of vanadium ion in the compound is:.
Audio playback is not audible. Question 2 Medium. Greene University of Ala… General Chemistry…. Career Point Career Point. Arihant Sanubia Salim, Yukta Khatri. Have you? In this case, M is given as 1. The angular momentum vector associated with an atomic state can take up only certain specified directions in space. Electrons in various suborbits of an filled in increasing order to their energies. Forgot Password? Sign up Login. See more 1-on-1 discussions 1. Chemistry Cengage Learning Cengage Learning Start Test. The atoms which are electrically neutral but have a magnetic moment, are formed into a narrow beam as they pass through a slit in a screen.

0 thoughts on “A compound of vanadium has a magnetic moment”