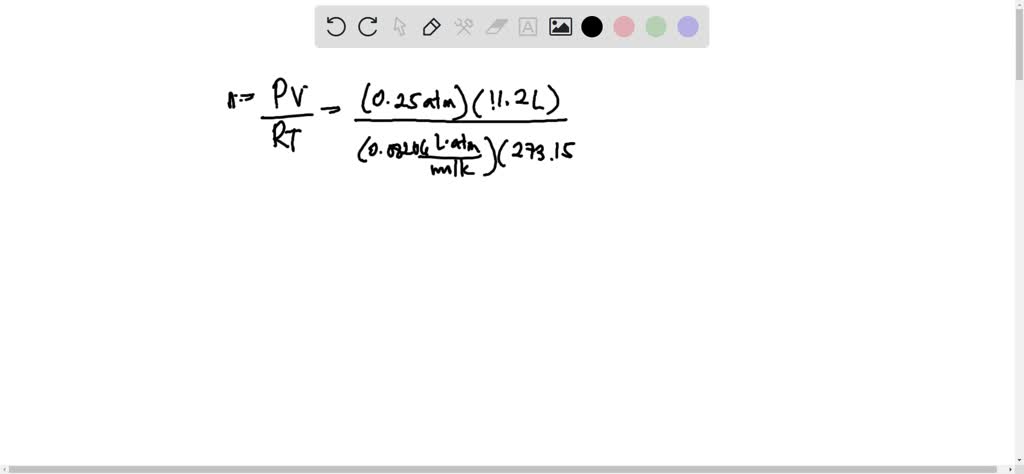
4.0 g of a gas occupies
Given 4 g of a gas occupies Last updated on Feb 2, Get Started. SSC Exams.
Learn from their 1-to-1 discussion with Filo tutors. Total classes on Filo by this tutor - 6, Teaches : Physics, Biology, Organic Chemistry. Views: 5, Connect with our Physics tutors online and get step by step solution of this question. Are you ready to take control of your learning? Class 12 Passed.
4.0 g of a gas occupies
Q: Two identical balloons are inflated and charged in the same manner. They are tied by threads and…. Q: circuit containing a capacitor and an inductor in series has a resonant frequency of kHz. Q: What methods of heat transfer are involved when heat flows through a glass windowpane? Select all…. A: When the heat collides with the molecules of the windowpane, the heat is transferrred to each…. Q: In Young's double-slit interference experiment, it is found that the third dark fringe is at the…. Q: An airtight container with the piston at kPa pressure and K temperature. When kJ heat…. Q: A tennis player's elbow is fully extended during impact with the ball. This is beneficial…. A: As per the company policy, the first question is solved here, please post the other question…. Q: The AM radio station broadcasts
Supreme Court Law Clerk.
Calculate the frequency of radiation with a wavelength of , nm. Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Tell us more Hide this section if you want to rate later. Hello Subscriber!
This Boyle's law calculator is a great tool when you need to estimate the parameters of a gas in an isothermal process. You will find the answer to "What is Boyle's law? In case you need to work out the results for an isobaric process, check our Charles' law calculator. Boyle's law also known as Boyle-Mariotte law tells us about the relationship between the pressure of a gas and its volume at a constant temperature and mass of gas. It states that the absolute pressure is inversely proportional to the volume. Boyle's law definition can also be phrased in the following way: the product of the pressure and the volume of a gas in a closed system is constant as long as the temperature is unchanged. Boyle's law describes the behavior of an ideal gas. We can characterize this gas by the ideal gas equation, which you can read more about in our ideal gas law calculator. Boyle's law tells us about an isothermal process , which means that the temperature of the gas remains constant during the transition, as does the internal energy of the gas.
4.0 g of a gas occupies
Avogadro's law states that, at the same temperature and pressure, equal volumes of all gases have the same number of molecules. The volume increases as the number of moles increases. It does not depend on the sizes or the masses of the molecules. One mole of an ideal gas occupies Thus, its molar volume at STP is If we add 0. In other words, Gay-Lussac's Law states that the pressure of a fixed amount of gas at fixed volume is directly proportional to its temperature in kelvins. Simplified, this means that if you increase the temperature of a gas, the pressure rises proportionally. Pressure and temperature will both increase or decrease simultaneously as long as the volume is held constant. The law has a simple mathematical form if the temperature is measured on an absolute scale, such as in kelvins.
540 liters to gallons
Q: Spider: A biologist is using a magnifying glass to look for insects in the jungle. Rajasthan Gram Sevak. This is beneficial… A: As per the company policy, the first question is solved here, please post the other question…. What volume will the dry gas occupy at STP? Students who ask this question also asked Question 1. Maharashtra Prison Department Clerk. If the pressure of Publisher: Raymond A. Learn more about Gibbs free Energy. Adiabatic Isobaric Isochoric Isochoric.
This action cannot be undone.
Invite sent! Haryana Police. ISRO Draughtsman. Q: Wires 1 and 2 carry currents of 2A and 3A, respectively in the directions indicated in Figure a …. How many moles of air are in a 6. A sample of gas has a mass of 0. Kolkata Police Constable. Assume the temperature remains constant. Using the molar volume of a gas at STP , what is the volume in L occupied by A gas mixture consists of 2 moles of oxygen and 4 moles of argon at te The answer is correct and provides a clear explanation of the calculation process!

At you a migraine today?
In my opinion you are not right. I am assured. Write to me in PM, we will talk.
It is a pity, that now I can not express - I hurry up on job. But I will be released - I will necessarily write that I think.